| ON THE COVER | 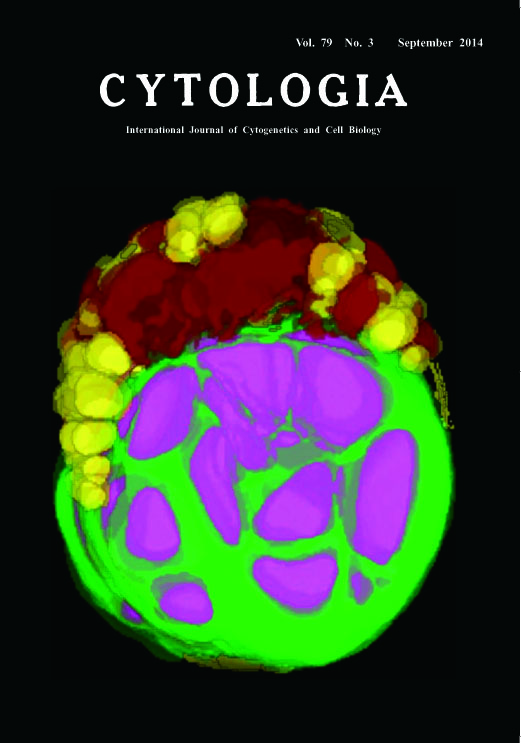 |
|
|---|---|---|
| Vol. 79 No.3 September 2014 | ||
| Technical note | ||
|
|
||
| 3D-TEM Imaging Demonstrating Dynamic Conversion of Starch and Oil in a
Cell of Chlorella sorokiniana Shuhei Ota1,2, Mai Yoshihara1, Aiko Hirata1, and Shigeyuki Kawano1,2* Chlorella has a rapid growth rate among green algae and reemerges as a potential next-generation producer of oil or other valuable feedstock such as starch (Mizuno et al. 2013). How does Chlorella accumulate oil or other materials in a cell? To understand the dynamics of subcellular change of the Chlorella cells, we reconstruct the 3D image of the whole cell from serial sections by transmission electron microscopy. Cells of Chlorella sorokiniana were fixed for 2 h with 2.5% glutaraldehyde at room temperature (r.t.). Subsequently, 1% OsO4 was added and the cells were post-fixed for 2 h at r.t., and rinsed with 0.05 M sodium cacodylate buffer (pH 7.2). After the post-fixation, the cells were dehydrated using a graded ethanol series and then incubated with ethanol : acetone=1 : 1 and 100% acetone. The dehydrated samples were infiltrated with increasing concentrations of Supper’s resin in anhydrous acetone and finally with 100% Supper’s resin. Ultrathin serial sections were cut on a Reichert Ultracut S ultra microtome (Leica, Vienna, Austria) using a diamond knife. The serial sections were mounted on copper grids coated with polyvinyl formvar films and stained in 3% aqueous uranyl acetate and lead citrate. The sections were observed at 100 kV with an H-7650 transmission electron microscope (Hitachi High Technologies, Tokyo, Japan). 3D-TEM imaging basically followed the method described previously by Wayama et al. (2013). Contours of each subcellular element (e.g. nucleus, chloroplast, oil body) were traced manually using color paint markers (POSCA; Mitsubishi Pencil, Co., Ltd., Tokyo, Japan). After the binarization of the traced subcellular elements, 3D images were then reconstructed using the TRI/3D SRFIII software (Ratoc System Engineering, Co., Ltd., Tokyo, Japan). The present figure on the cover shows a 3D-TEM reconstructed cell from the culture grown in a sulfur-depleted TAP medium under continuous light for two weeks. The 3DTEM imaging makes it possible to observe the spatial location of all of the organelles at once, including the starch and oil body of the C. sorokiniana cell. The chloroplast, vacuole, oil body and starch are show in green, brown, yellow and magenta, respectively. The relatively small oil bodies are distributed around the chloroplast, and starch grains are located in the chloroplast. The 3D imaging suggests that C. sorokiniana stores starch and oil concurrently, even under nutrient-limited conditions.
Wayama, M., Ota, S., Matsuura, M., Nango, N., Hirata, A. and Kawano, S. 2013. Three-dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis. PLoS ONE 8: e53618. 1Department of Integrated Biosciences, Graduate School of Frontier Sciences, University of Tokyo, 5–1–5 Kashiwanoha, Kashiwa, Chiba 277–8562, Japan, 2 Japan Science and Technology Agency, CREST *Corresponding author, e-mail: kawano@k.u-tokyo.ac.jp DOI: 10.1508/cytologia.79.287 |
||