| ON THE COVER | 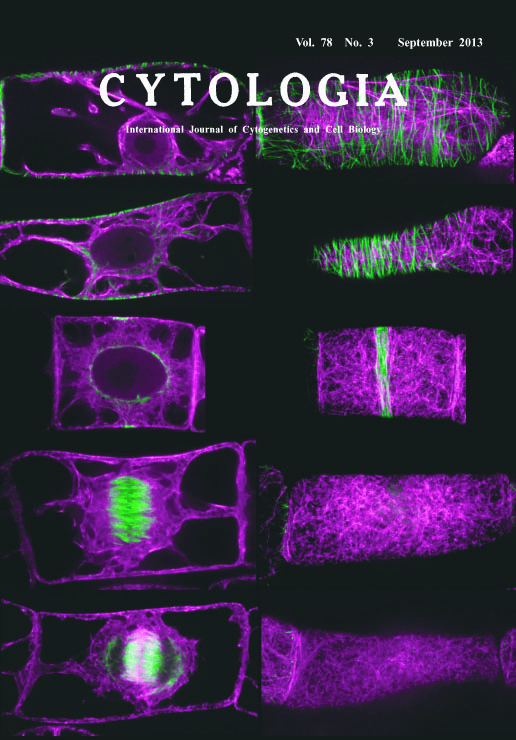 |
|---|---|
| Vol. 78 No.3 September 2013 | |
| Technical note | |
|
|
|
| Dual Observation of Microtubules and Actin Microfilaments during Cell Cycle
Progression in BY-YTRF1 Cells Microtubules and actin microfilaments play essential roles in wide variety of cellular activity in eukaryote. Classically, these cytoskeletal components have been visualized by fluorescent labeling in chemically fixed cells. Although these approaches are still useful in non-model organisms that unable to efficient transformation, these cytoskeletal components are sensitive in chemical fixation and so the artificial organization of cytoskeleton was frequently induced in chemically fixed cells. Visualization of microtubules or actin microfilaments with fluorescent protein have revealed more intact cytoskeletal organization in living tobacco BY-2 cells. However, BY-2 cells visualized both microtubules and actin microfilaments with fluorescent protein have not been established. To observe microtubules and actin microfilaments simultaneously in living BY-2 cells, we established a transgenic BY-2 cells stably expressing YFP-tubulin and tdTomato-ABD2, designated as BY-YTRF1 cells. These cells showed similar growth rate and mitosis progression by aphidicolin treatment to the original BY-2 cells. The image on the title page shows microtubules (illustrated as green) and actin microfilaments (illustrated as magenta) at G1 phase (1st row from the top), S phase (2nd row), late G2 phase (3rd row), metaphase (4th row) and telophase (5th row). Images in the left and right column show cell mid-plane and maximum intensity projections near the cell cortex, respectively. As representative cytoskeletal structures, the cortical microtubules and actin microfilament from G1 to S phase, the preprophase band at late G2 phase, mitotic spindle and cortical actin microfilament patterning called actindepleted zone or actin microfilament twin peaks at metaphase, and the phragmoplast at telophase could be observed. Using the BY-YTRF1 cells, we recently revealed that the disordered cortical actin microfilament patterning is correlated with an oblique mitotic spindle orientation and resulted in an oblique division plane, suggesting that cortical actin microfilament patterning regulates the spindle orientation (Kojo et al. 2013, Plant and Cell Physiology 54: 1491-1503). In conclusion, we newly established BY-YTRF1 cells and confirmed that these cells allowed the simultaneous observation of microtubule and actin microfilament components during cell cycle progression in living cells. (Kei H. Kojo1, Hiroki Yasuhara2, Nastumaro Kutsuna1, and Seiichiro Hasezawa1,3*, 1 Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha Kashiwa, Chiba 277-8562, Japan. 2 Department of Life Science and Biotechnology, Faculty of Chemistry, Materials and Bioengineering, Kansai University, 3-3-35 Yamate-cho, Suita, Osaka 564-8680, Japan. 3 Advanced Measurement and Analysis, Japan Science and Technology Agency (JST), Chiyoda-ku, Tokyo 102-8666, Japan.) |
|